Julie Appleby, Kaiser Health News and Elizabeth Lucas, Kaiser Health News
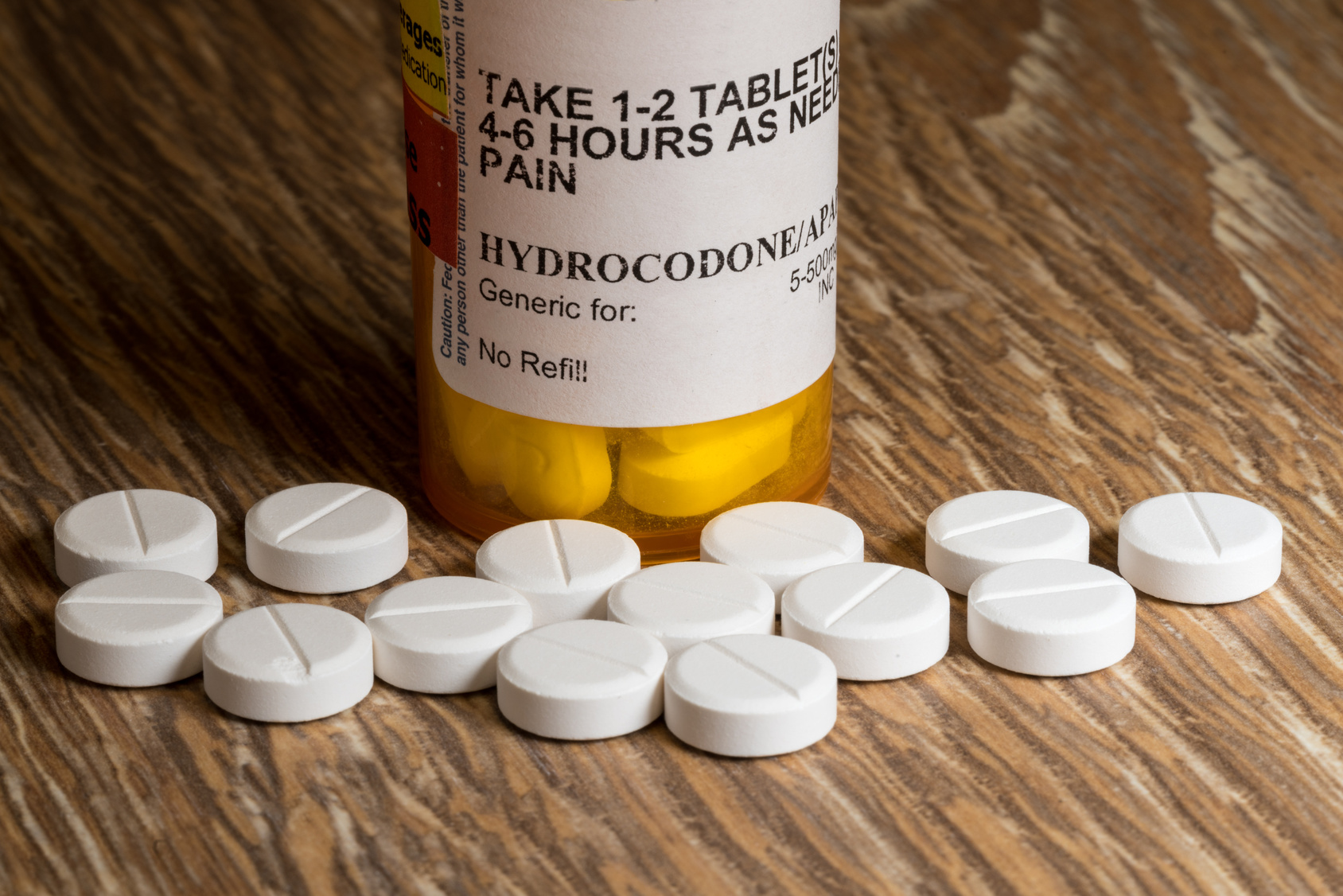
As opioid addiction and deadly overdoses escalated into an epidemic across the U.S., thousands of surgeons continued to hand out far more pills than needed for postoperative pain relief, according to a KHN-Johns Hopkins analysis of Medicare data.
Many doctors wrote prescriptions for dozens of opioid tablets after surgeries — even for operations that cause most patients relatively little pain, according to the analysis, done in collaboration with researchers at Johns Hopkins Bloomberg School of Public Health. It examined almost 350,000 prescriptions written for patients operated on by nearly 20,000 surgeons from 2011 to 2016 — the latest year for which data are available.
Some surgeons wrote prescriptions for more than 100 opioid pills in the week following the surgery. The total amounts often exceeded current guidelines from several academic medical centers, which call for zero to 10 pills for many of the procedures in the analysis, and up to 30 for coronary bypass surgery.
While hundreds of state and local lawsuits have been filed against opioid manufacturers, claiming they engaged in aggressive and misleading marketing of these addictive drugs, the role of physicians in contributing to a national tragedy has received less scrutiny. Research shows that a significant portion of people who become addicted to opioids started with a prescription after surgery.
In sheer numbers, opioid prescribing in the U.S. peaked in 2010, but it remains among the highest in the world, according to studies and other data.
In 2016, opioids of all kinds were linked to 42,249 deaths, up from the 33,091 reported in 2015. The opioid-related death rate jumped nearly 28% from the year before, according to the CDC.
Yet long-ingrained and freewheeling prescribing patterns changed little over the six years analyzed. KHN and Johns Hopkins examined the prescribing habits of all U.S. surgeons who frequently perform seven common surgical procedures and found that in the first week after surgery:
- Coronary artery bypass patients operated on by the highest-prescribing 1% of surgeons filled prescriptions in 2016 exceeding an average of 105 opioid pills.
- Patients undergoing a far less painful procedure — a lumpectomy to remove a breast tumor — were given an average of 26 pills in 2016 the week after surgery. The highest-prescribing 5% of surgeons prescribed 40 to 70 pills on average.
- Some knee surgery patients took home more than 100 pills in the week following their surgery.
Those amounts — each “pill” in the analysis was the equivalent of 5 milligrams of oxycodone — are many times what is currently recommended by some physician groups to relieve acute pain, which occurs as a result of surgery, accident or injury. The analysis included only patients not prescribed opioids in the year before their operation.
“Prescribers should have known better” based on studies and other information available at the time, said Andrew Kolodny, co-director of opioid policy research at Brandeis University and director of the advocacy group Physicians for Responsible Opioid Prescribing.
While the dataset included only prescriptions written for patients on Medicare, the findings may well understate the depth of the problem, since doctors are more hesitant to give older patients the powerful painkillers because of their sedating side effects.
Surgeons’ prescribing habits are significant because studies show that 6% of patients who are prescribed opioids after surgery will still be taking them three to six months later, having become dependent. The likelihood of persistent use rises with the number of pills and the length of time opioids are taken during recuperation.
Also, unused pills in medicine cabinets can make their way onto the street.
Dr. Marty Makary, a surgical oncologist at Johns Hopkins, admits that he too once handed out opioids liberally. Now he is marshaling a campaign to get surgeons to use these powerful painkillers more consciously and sparingly. “I think there’s an ‘aha’ moment that many of us in medicine have had or need to have,” he said.
But old habits are hard to kick.
KHN contacted dozens of the surgeons who topped the ranks of opioid prescribers in the 2016 database. They hailed from small, community hospitals as well as major academic medical centers. The majority declined to comment, some bristling when questioned.
Look Up Opioid Prescribers: Search KHN Database By Doctor, Hospital
Some of those surgeons were critical of the analysis, saying it didn’t take into account certain essential factors. For example, it was not possible to determine whether patients had complications or needed higher amounts of pain medication for another reason. And some surgeons had only a handful of patients who filled prescriptions, making for a small sample size.
But surgeons also indicated that the way they prescribe pain pills was less than intentional. It was sometimes an outgrowth of computer programs that default to preset amounts following procedures, or practice habits developed before the opioid crisis. Additionally, they blame efforts in the late 1990s and early 2000s that encouraged doctors and hospitals to consider pain as “the fifth vital sign.” A major hospital accrediting group required providers to ask patients how well their pain was treated. Pharmaceutical companies used the fifth vital sign campaign as a way to promote their opioid treatments.
Makary, who oversaw the analysis of the Medicare dataset, said that, while opioid prescribing is slowly dropping, to date many surgeons have not paid enough attention to the problem or responded with sufficient urgency.
Dr. Audrey Garrett, an oncologic surgeon in Oregon, said she was “surprised” to hear that she was among the top tier of prescribers. She said she planned to re-evaluate her clinic’s automated prescribing program, which is set to order specific amounts of opioids.
KHN will analyze data for 2017 and subsequent years when it becomes available to follow how prescribing is changing.
Prescribing Patterns Highlight What’s At Stake
The analysis examined prescribing habits after seven common procedures: coronary artery bypass, minimally invasive gallbladder removal, lumpectomy, meniscectomy (which removes part of a torn meniscus in the knee), minimally invasive hysterectomy, open colectomy and prostatectomy.
Across the board, the analysis showed that physicians gave a large number of narcotics when fewer pills or alternative medications, including over-the-counter pain relief tablets, could be equally effective, according to recent guidelines from Makary and other academic researchers.
On average, from 2011 to 2016, Medicare patients in the analysis took home 48 pills in the week following coronary artery bypass; 31 following laparoscopic gallbladder removal; 28 after a lumpectomy; 41 after meniscectomy; 34 after minimally invasive hysterectomy; 34 after open colon surgery; and 33 after prostatectomy.
According to post-surgical guidelines spearheaded by Makary for his hospital last year, those surgeries should require at most 30 pills for bypass; 10 pills for minimally invasive gallbladder removal, lumpectomy, minimally invasive hysterectomy and prostatectomy; and eight pills for knee surgery. It has not yet published a guideline for open colon surgery.
The Johns Hopkins’ doctors developed their own standards because of a dearth of national guidelines for post-surgical opioids. They arrived at those figures after reaching a consensus among surgeons, nurses, patients and other medical staff on how many pills were needed after particular surgeries.
Hoping to reduce overprescribing, Makary is preparing to send letters next month to surgeons around the country who are among the highest opioid prescribers under a grant he received from the Arnold Foundation, a nonprofit group whose focus includes drug price issues. (Kaiser Health News also received funding from the Arnold Foundation.)
Even if the prescription numbers have fallen since 2016, the amounts given today are likely still excessive.
“When prescribing may have been five to 20 times too high, even a reduction that is quite meaningful still likely reflects overprescribing,” said Dr. Chad Brummett, an anesthesiologist and associate professor at the University of Michigan.
Brummett is also co-director of the Michigan Opioid Prescribing Engagement Network, a collaboration of physicians that makes surgery-specific recommendations, many of them in the 10- to 20-pill range.
“Reducing unnecessary exposure is key to reducing the risk of new addiction,” said former Food and Drug Administration commissioner Scott Gottlieb. In August 2018, when Gottlieb was at the agency’s helm, it commissioned a report from the National Academy of Sciences on how best to set opioid prescribing guidelines for acute pain from specific conditions or surgical procedures. Its findings are expected later this year.
“There are still too many 30-tablet prescriptions being written,” said Gottlieb.
Healers Sowing Disease?
Naturally, surgeons rankle at the idea that they played a role in the opioid epidemic. But studies raise serious concerns.
Transplant surgeon Dr. Michael Engelsbe, director of the Michigan Surgical Quality Collaborative, points to the study showing 6% of post-op patients who get opioids for pain develop long-term dependence. That means a surgeon who does 300 operations a year paves the way for 18 newly dependent people, he said.
Many patients do not need the amounts prescribed.
Intermountain Healthcare, a not-for-profit system of hospitals, clinics, and doctors in Utah, began surveying patients two years ago to find out how much of their prescribed supply of opioids they actually took following surgery.
“Globally, we were overprescribing by 50%,” said Dr. David Hasleton, senior medical director.
But Intermountain approached individual doctors carefully. “If you go to a prescriber to say, ‘You are overprescribing,’ it never goes well. A common reaction is, ‘Your data is wrong’ or ‘My patients are different than his,’” said Hasleton.
For the analysis, KHN attempted to contact more than 50 surgeons whose 2016 numbers ranked them among the top prescribers in each surgical category.
One who did agree to speak was Dr. Daniel J. Waters, who 13 years ago had his chest cut open to remove a tumor, an operation technically similar to what he does for a living: coronary artery bypass.
“So I have both the doctor perspective and the patient perspective,” said Waters, who practices in Mason City, Iowa.
In 2016, Waters’ Medicare bypass patients who filled their prescriptions took home an average of nearly 157 pills each, according to the KHN-Johns Hopkins analysis.
“When I went home from the hospital, 30 would not have been enough,” said Waters of the number recommended by the Hopkins team for that surgery.
But he said he has recently curbed his prescribing to 84 pills.
Nationally, the average prescription filled for a coronary artery bypass was 49 pills in 2016 and had changed little since 2011, the analysis shows.
Others who spoke with KHN said they had developed the habit of prescribing copiously — sometimes giving out multiple opioid prescriptions — because they didn’t want patients to get stuck far from the office or over a weekend with pain or because they were trying to avoid calls from dissatisfied, hurting patients.
In the KHN-Johns Hopkins data, the seven patients of Dr. Antonio Santillan-Gomez who filled opioid prescriptions after minimally invasive hysterectomies in 2016 received an average of 77 pills each.
A gynecologic oncologist, Santillan-Gomez said: “I’m in San Antonio, and some of my patients come from Laredo or Corpus Christi, so they would have to drive two to three hours for a prescription.”
Still, he said, since e-prescribing of opioids became more widespread in the past few years, he and other surgeons in his group have limited prescriptions to 20 to 30 pills and encouraged patients to take Tylenol or other over-the-counter medications if they run out. E-prescribing can not only help track patients getting opioids but also reduce the problem of patients having to drive back to the office to get a written prescription.
Dr. Janet Grange, a breast surgeon in Omaha, Neb., said that in her experience, opioid dependence had not been a problem.
“I can absolutely tell you I don’t have even 1% who become long-term opioid users,” said Grange.
The analysis showed that Grange had 12 opioid-naïve Medicare patients who had a lumpectomy in 2016. Eight of them filled prescriptions for an average of 47 pills per patient.
She called Johns Hopkins’ zero-to-10-pill pain-control recommendation following that procedure “miserly.”
The Pendulum Swings
Some of the higher-prescribing surgeons in the KHN-Johns Hopkins analysis reflected on their potential contribution to a national catastrophe and are changing their practice.
“That is a shocking number,” said oncologist Garrett, speaking of the finding that 6% of patients who go home with opioids will become dependent. “If it’s true, it’s something we need to educate physicians on much earlier in their medical careers.”
Garrett, in Eugene, Ore., said she has cut back on the number of pills she gives patients since 2016. The KHN-Johns Hopkins analysis showed that seven of her 13 opioid-naïve Medicare patients undergoing minimally invasive hysterectomies filled a prescription for opioids in 2016. Those patients took home an average of 76 pills each.
Johns Hopkins guidelines call for no more than 10 opioid pills following this procedure, while Brummett’s Michigan network recommends no more than 15.
Surgeon and researcher Dr. Richard Barth, once a heavy prescriber himself, said that his own experience convinced him that physicians’ preconceptions about how much pain relief is needed are often way off.
The analysis showed his lumpectomy patients in 2013 filled an average of 33 pills in the week after surgery. By 2016, that average had dropped to seven pills. Many patients, he said, can do just fine after lumpectomy with over-the-counter medications — and often no opioids at all.
The key, he said, is to set patients’ expectations upfront.
“I tell them it’s OK to have a little discomfort, that we’re not trying to get to zero pain,” said Barth, who is chief of general surgery at Dartmouth-Hitchcock Medical Center and has published extensively on opioid prescribing.
After lumpectomy, “what I recommend is Tylenol and ibuprofen for at least a few days and to use the opioids only if the discomfort isn’t relieved by those.”
Indeed, the data analysis showed that a significant number of patients given prescriptions for opioids never filled them because they don’t need that level of pain relief.
Between 2011 and 2016, for example, only 62% of lumpectomy patients in the analysis filled prescriptions, similar to hysterectomy patients.
In 2016, patients of Dr. Kimberli Cox, a surgeon in Peoria, Ariz., were prescribed about 59 pills in the week following lumpectomy, well above the recommendations from both Johns Hopkins and others.
But the KHN-Johns Hopkins analysis of that year’s data shows that half of her patients never filled a painkiller prescription — a fact she acknowledges has changed her thinking.
“I am now starting to prescribe less because many patients say, ‘You gave me too many’ or ‘I didn’t fill it,” she said.
Disclaimer: The viewpoint expressed in this article is the opinion of the author and is not necessarily the viewpoint of the owners or employees at Healthcare Staffing Innovations, LLC.